
Various strategies to cure diabetes have been evaluated over the years with limited success. Remarkably, this chimeric capsid also enabled transduction of both mouse and human hepatocytes at very high levels in a humanized chimeric mouse model, thus providing a versatile vector that has the potential to be used in both preclinical testing and human clinical trials for liver-based diseases and diabetes.Īn estimated 30.3 million US Americans are affected by either type 1 or type 2 diabetes mellitus ( 1). We describe the generation of a chimeric AAV capsid (AAV-KP1) that facilitates transduction of primary human islet cells and human embryonic stem cell–derived β cells with up to 10-fold higher efficiency compared with previously studied best-in-class AAV vectors. Because of the recent success in reprogramming islet-derived cells into functional β cells in animal models, we constructed 2 highly complex barcoded replication competent capsid shuffled libraries and selected for high-transducing variants on primary human islets. ( e,f) Stereological counts of GFP-labeled cells and area of spread after each vector show that AAV2 shows greater transduction efficiency over a larger volume than chimeric-1829 in these areas.While gene transfer using recombinant adeno-associated viral (rAAV) vectors has shown success in some clinical trials, there remain many tissues that are not well transduced. ( d) Comparison of the transduction efficiency of AAV2 and chimeric-1829 after injections into the ( a and c) caudate nucleus and ( b and d) substantia nigra of monkeys after immunostaining for green fluorescent protein (GFP). ( c) Patterns of transduction in the rat striatum 1 month after a 1 μl infusion of either AAV1829 or AAV2. Bioluminescent images were obtained at the end of 1 month using a Xenogen IVIS system. ( b) Luciferase transgene expression in the liver through tail vein injecton of 10 11 vector genomes of the novel AAV variant and parental AAV1, 2. ( a) Luciferase transgene expression in the hindlimbs of Balb/C mouse injected with 10 10 vector genomes of the novel adeno-associated virus (AAV) variant and parental AAV1, 2. ( b) Dot blot demonstrating the directed evolution of cell type–specific AAV variant from CS1 cells after five infectious cycles ( n = 5). Subsequent steps will involve development of novel AAV variants as targeted vectors for in vivo gene delivery applications. Specific cell line (hamster melanoma CS1 cells) previously shown to be restricted to transduction of AAVs will be tested with the chimeric AAV library for a premissive clone. AAV particle library was generated by plasmid transfection protocol. Following this reassembly reaction, PCR amplification with specific primers was used to generate full-length Cap chimeras for cloning into wild-type AAV2 backbone to generate shuffled plasmid library. AAV Cap genes were randomly fragmented and reassembled using cycles of denaturation, annealing, and extension using PCR.
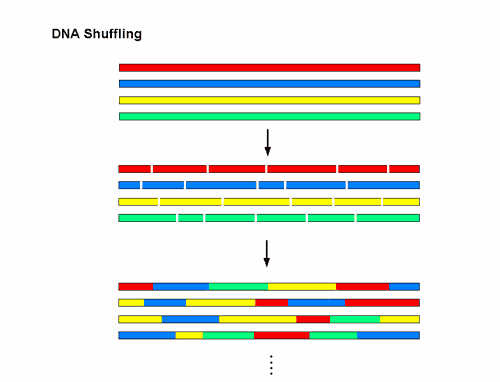
( a) Strategy for generating a combinatorial adeno-associated virus (AAV) library that will be subjected to directed evolution to isolate cell type/receptor-specific AAV mutants. Application of this technology to alternative cell/tissue types using AAV or other viral capsid sequences is likely to yield a new class of biological nanoparticles as vectors for human gene transfer. We determined a unique immunological profile based on neutralizing antibody (NAb) titer and crossreactivity studies strongly supporting isolation of a synthetic laboratory-derived capsid variant. Further, chimeric-1829 demonstrates altered tropism in rodent skeletal muscle, liver, and brain including nonhuman primates.
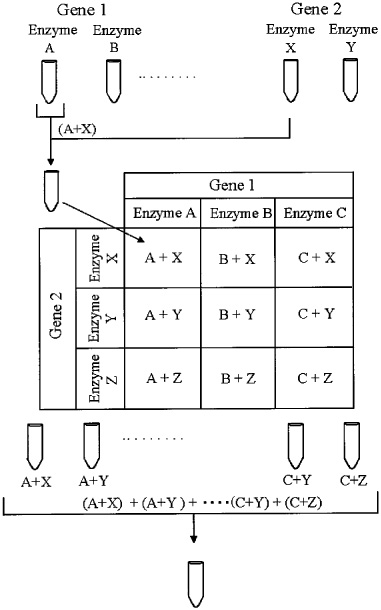
Chimeric-1829 utilizes heparan sulfate as a primary receptor and transduces melanoma cells more efficiently than all serotypes. The C-terminal domain (AAV9) was identified as a critical structural determinant of melanoma tropism through rational mutagenesis. Molecular modeling studies suggest that AAV2 contributes to surface loops at the icosahedral threefold axis of symmetry, while AAV1 and 9 contribute to two- and fivefold symmetry interactions, respectively. A single infectious clone (chimeric-1829) containing genome fragments from AAV1, 2, 8, and 9 was isolated from an integrin minus hamster melanoma cell line previously shown to have low permissiveness to AAV. Capsid genomes of adeno-associated virus (AAV) serotypes 1-9 were randomly fragmented and reassembled using PCR to generate a chimeric capsid library. We report a DNA shuffling-based approach for developing cell type-specific vectors through directed evolution.


 0 kommentar(er)
0 kommentar(er)
